The ‘Disappearance’ of Polio (III)
A story about how to vaccinate people against a friendly pathogen which helps humans to aquire natural immunity, and about vaccine production and vaccine approval despite harmful effects
Read the second part of the article
Looking beyond Cutter
Here is some of what Paul Offit left out of his book. Even though Cutter Laboratories took the fall for the 1955 disaster, all manufacturers had difficulty killing the virus in their vaccines before and after the disaster. Cutter was not the only manufacturer documented to have produced live virus vaccine that was injected into children and caused paralysis. In 1990, after decades of information concealment, the Freedom of Information Act led to the release of documents that proved Wyeth also produced a paralyzing vaccine.
Wyeth and Cutter are thought today to have been the only companies that produced live virus vaccine; however, all the vaccine companies could have released active vaccine virus because the “minimum licensing requirements” set by the US Department of Health, Education, and Welfare were not met by any pharmaceutical company. The initial minimum licensing requirements established in April 12, 1955, stated that “all virus infectivity is destroyed with certainty.” According to later documents and courtroom testimonies, this definition was not followed, and manufacturers were never held to such standards. In 1992 Dr. Neil Nathanson stated: “Minimum requirements were meant to state the assurance that the final vaccine contained less than 5 tissue culture infectious doses per liter … in other words to assure that there would be less than one chance in 100,000 that the vaccine would contain one paralytogenic dose per 1,000 human doses of vaccine.”
Does this sound like insurance that “all infectivity was destroyed with certainty?” A Tissue Culture Infective Dose (TCID) is a mathematical calculation. According to the late virologist Dr. Wendell Stanley, a single TCID contained up to 30 poliovirus particles, and any one of them could have caused poliomyelitis.
There are a couple of problems associated with risk calculation using TCIDs. First, the cutoff choice of less than 5 TCIDs was arbitrary. Second, there is an assumption that all virions (complete, infectious virus particles) would be distributed evenly and necessarily be included in any test sample. According to statistician Dr. Paul Meier, if each virion injected did cause a case of paralytic poliomyelitis, the injection of 1 milliliter of vaccine where the batches contain 5 TCIDs per liter could cause up to 500 cases per 100,000 vaccinated.
The reason there was not much more paralysis among the vaccinated was because, as was already known, 80-90 percent of the childhood population at the time was already naturally immune to at least one strain of poliovirus. In his book, Dr. John Paul estimated that 80 percent would have had some pre-vaccine antibody to the poliovirus. Anyone who was immune naturally would also, fortunately, have been immune to the corresponding vaccine virus.
You may be wondering how this information was concealed from the public for nearly fifty years. Congressman Percy Priest ordered and chaired a full investigation of the vaccine controversy.
He admitted in 1956 that: “… in the previous year (1955) many responsible persons had felt that the public should be spared the ordeal of ‘knowledge about controversy.’ If word ever got out that the Public Health Service had actually done something damaging to the health of the American people, the consequences would be terrible…. We felt that no lasting good could come to science or the public if the Public Health Services were discredited.”
So much for evidence-based medicine and scientific truth. Instead of discrediting the PSU, the decision was made, after some deliberation, to leave Wyeth’s paralyzing vaccine on the market, place the whole blame on Cutter, and ignore the ongoing problem with live viruses in the vaccines that persisted even after the revisions for safer manufacture were carried out. Only Cutter’s vaccines were recalled. All other manufacturers’ vaccines released in the 1950s were sold and injected into America’s children. Millions of vaccines were also exported all around the world.
There were other more insidious and unaddressed problems with the Salk vaccine. Once a vaccine passed the minimum requirement tests showing that all the virus was theoretically killed, the virus was found to have resurrected on the shelves weeks or months later, even after the new safety standards were put in place in 1956. “Dr. S Stephen Chapman … reported … he had centrifuged the vaccine and had obtained live virus, ‘more than we theoretically ever could have anticipated having … this brings up the problem of reactivation of the so called dead vaccine.’“
The most likely explanation for this apparent resurrection is that the safety testing didn’t detect small amounts of live virus, and without Merthiolate in the vaccine the virus was able to replicate. In 1954 Salk’s trial vaccine contained a mercury compound patented as Merthiolate, which was used to prevent mold from growing and to prolong the shelf life. When it was obvious that the vaccine was not as antigenic as hoped, a decision was made to remove the mercury compound in the 1955 manufacture. Salk never wanted the mercury in the first place and protested that it ruined the vaccine, making the Mahoney strain less antigenic.
Swedish scientists, after the Cutter disaster in 1955, began to test some of their vaccine that was waiting to be dispensed. They did this in response to the alarming news coming from the United States about vaccine-induced paralysis. Tests were done on batches of vaccine that were previously shown to be free of active virus. Upon repeat testing, 30% of the vaccine samples showed the presence of active virus.
The fundamental problem was that, although required safety testing was done with the hope of releasing only safe vaccines, the foundational principles with which the vaccine was manufactured were highly flawed from the beginning. Salk’s hypothesis was false. This problem was never fully addressed.
According to expert virologist Dr. Sven Gard, a fundamental property of the virus that had to do with its structure was overlooked. Dr. Gard also stated that vaccination in the United States caused as many cases of poliomyelitis as it prevented in 1955.
Dr. Edwin Lennette, director of the California State Department of Health stated that, in general, vaccines could test negative in the lab and in test animals, yet behave differently in humans: “You just put in some formaldehyde or whatever and inactivate the virus, and you do a few tests, and if nothing happens in the animal, then you think, well, we’ve got a vaccine. But you put it into man, who is the ultimate susceptible animal, and then something else goes wrong, and you’ve got a problem.”
In subsequent years, instead of removing the dangerous Mahoney strain, American manufacturers continued releasing vaccines that were safer but far less antigenic. They tended to the problem, not by addressing the fundamental flaw, but by adding more filtrations of the vaccine. Even with such effective viral dilution of the vaccine and four revisions to the minimum requirements set forth by the government for producing safe vaccines in 1955, there was ongoing evidence that vaccine-induced infections continued. There were preseason polio (as in vaccine-provoked polio) peaks that were not present before the vaccine years.
Not all the vaccine-induced cases were accepted by the Polio Surveillance Unit. Many paralyzed recipients were denied validation and compensation for illness that occurred after the vaccine was given in 1955. The requirements for so-called accepted cases of vaccine-associated polio were more stringent than the requirements for reporting polio in nonvaccinated individuals.
The Salk vaccine was anything but a lifesaver. It was known from the start to be trouble, and trouble it was. Wild poliovirus was never a lone or major cause of poliomyelitis. But even if it was, the Salk vaccine could not possibly have been a solution to ridding the world of polio. Nonetheless, Jonas Salk and his vaccine have been forever cast into heroism in the archives of vaccine mythology.
Monkey virus (SV40) contamination
Vaccines manufactured using monkey kidneys up into the 1980s have been definitively noted to contain a carcinogenic monkey virus that some medical researchers believe can result in cancer in a portion of the millions who were given them. Simian virus number 40 (SV40) is a monkey virus that has been found in several types of human cancers, including lung mesotheliomas, several types of brain tumors, and bone, breast, colon, and kidney tumors. Unfortunately, the controversy over the percentage of tumor specimens containing SV40 DNA and proteins has paralyzed the research field. Because of financial and political conflicts of interest, the research necessary to firmly validate the vaccine-virus association will probably never be done.
Monkey cell cultures are still used in polio vaccine production today. According to Stanley Kops’ allegations, SV40 was and still is a potential risk in both the OPV and the inactivated polio vaccine (IPV). The IPV used in the developed world is still treated with formaldehyde, but SV40 has been known since 1961 to survive formaldehyde beyond the usual 12-day minimum. Vaccine manufacturers today cite a minimum of 12 days of formaldehyde treatment.
History repeats itself
In India today, as the WHO tracks polio during the vaccination campaigns, reports of paralytic cases associated with wild-type poliovirus have declined, and AFP (acute flacid paralysis) has increased annually, reaching 60,000 new cases in 2011.
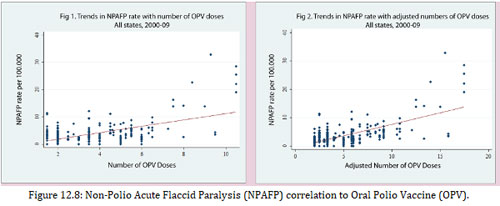
The causes of AFP that have been identified are as follows: “Poliomyelitis, non-polio enterovirus, vaccine-associated poliomyelitis (which can include polio vaccines), rabies virus, varicella zoster virus, Japanese encephalitis virus, Guillain-Barré syndrome, cytomegalovirus, sciatic neuritis from injection, transverse myelitis, epidural abscess, spinal cord compression, exotoxin of corynebacterium diptheriae, toxin of clostridium botulinum, Karwinskia, tick bite paralysis, Lyme borreliosis, myasthenia gravis, polymyositis autoimmune, viral myositis, trichinosis, toxic myopathies among others.”
In spite of (or perhaps because of) the aggressive OPV campaigns in India, there has been a steep ascent in AFP diagnoses. Nonetheless, the WHO and its sister organizations celebrate because the number of documented cases of wild poliovirus-associated paralysis has declined.
It just so happens that DDT is still heavily used in India. Despite the well-documented connection between poliomyelitis and DDT symptoms, including anterior horn spinal cord damage, respiratory paralysis, muscle spasm, and weakness, multi-billion dollar polio eradication campaigns march on. Often, an Indian child is vaccinated 15 times (or more) with live vaccine by age five.
A major oversight on the part of the press and the medical establishment as they observe the WHO’s version of history is that massive “pulse” vaccination campaigns have done nothing to eliminate childhood paralysis and, in fact, there is strong evidence pointing to the likelihood that experimental polio vaccination is related to the sharp rise in AFP.
It has been reported in the Lancet that the incidence of AFP, especially non-polio AFP, increased drastically in India after an experimental, high-potency polio vaccine was introduced. Worse still is that children identified with non-polio AFP are at more than twice the risk of dying than those with wild polio infection. Isn’t vaccination really about eliminating paralysis… or is it simply to replace wild virus with a vaccine virus regardless of the outcome?
Wild polioviruses, vaccine polioviruses, and neurovirulent Coxsackie viruses can all interact, recombine, and evolve into seriously neurovirulent entities. Why would a vaccine virus be stable and not follow the laws of nature, which involve the clear likelihood of recombination?
The response to the rise in AFP in India by the WHO and the Global Alliance for Vaccines and Immunisation (GAVI) has been to ramp up the oral polio vaccination campaigns in recent years. Now some children are reported to have received 32 vaccines by five years of age. In the past, there was never such an aggressive effort to inoculate children up to 30 times for one disease by their fifth birthday.
Just what are GAVI members trying to accomplish? Does it look like the sustainable health and betterment of India’s people are the main goals? Dr. V. I. Agol commented in Nature that vaccination against poliomyelitis might have to continue indefinitely.
Conclusion
By now it should be obvious that there was more to the “polio” story than a crippling virus and a world that was saved by a vaccine. Isn’t it strange that the reasoning behind polio epidemics in the United States in the 1940s was increased societal hygiene? Filth, back then, was thought to be protective against polio! The explanation given was that babies in areas with better hygiene (unlike the native people who were known to be immune without developing poliomyelitis) were not exposed to wild virus early enough due to societal cleanliness and therefore did not develop early natural immunity. Today India is told that paralytic poliovirus infections are a result of poor societal hygiene. Such doublespeak demonstrates how the tenet changes to accommodate the vaccine agenda and deny the true causes of paralysis.
The National Foundation for Infantile Paralysis was overseen by the major medical monopoly, the Rockefeller Institute. Vaccination continues as the sole intervention for the perceived problem of poliomyelitis in India and other undeveloped countries, even in the face of vaccine-induced paralysis, vaccine virus mutations, and obvious failures. When vaccine programs don’t live up to their promises, the blame is always placed on the unvaccinated, or a new angle is drawn to the tune of “five vaccines per child may not be enough.” By sleight of hand, changing the diagnosis of old-time polio to AFP, any ongoing paralysis will be covered while the dimes continue to roll in.
In addition to the rise in AFP that correlates with rising OPV dosing in India, there are numerous reports of vaccine viruses mutating to virulence, causing polio outbreaks in China, Nigeria, and India. As always, the finger is pointed at under-vaccinated populations rather than at the vaccine itself or the myriad other causes of viral mutation.
We often hear that OPV circulating in poorly immunized populations is wonderful because the unvaccinated get the benefit. But OPV vaccines will always be able to recombine with enteroviruses no matter how highly vaccinated the population, and dangerous recombination viruses that cause paralysis will not be called “polio.” This is one way that a mountain of new AFP cases builds, while GAVI and WHO celebrate eradication of polio.
If poliovirus is reintroduced into the toxic, unhealthy, immunologically naive population, from residual samples stored in laboratories, some of which are highly neurovirulent (recall 1916 New York City), circulating vaccine-derived polioviruses, or poliovirus that is chemically synthesized, the potential outcome is unfathomable.
Today children are forced to submit to vaccines because the WHO and others are just targeting wild poliovirus and not the problem of paralysis. Once this very shortsighted goal is met, there will undoubtedly be future trouble. The WHO knows this and already has considered the steps necessary to deal with the immunologically naive population if viral reintroduction occurs.
History books of the future may reflect upon a disaster with this conclusion: Wild poliovirus should have been left alone and the real sources of paralysis pursued and addressed.
