Side Effects of the Measles Vaccine Include Brain Damage and Death (1)
The Centers for Disease Control (CDC) report minor side effects from the MMR-V and MMR vaccines to include low-grade fever, injection site redness or rash, pain at the injection site, and facial swelling. Moderate side effects include a full body rash, temporary low platelet count, temporary stiffness and pain the joints and seizures, and seizures. MMR-V, however, has been noted to have a higher risk of seizures than separate administrations of MMR and varicella vaccines, especially when given as the first dose of the series.
Rare serious side effects of both MMR-V and MMR include brain damage, coma, chronic seizure disorder, lowered level of consciousness and loss of hearing.
Serious complications reported by Merck in the ProQuad (MMR-V) product insert during vaccine post-marketing surveillance include:
• measles;
• atypical measles;
• vaccine strain varicella;
• varicella-like rash;
• herpes zoster;
• herpes simplex;
• pneumonia and respiratory infection;
• pneumonitis;
• bronchitis;
• epididymitis;
• cellulitis;
• skin infection;
• subacute sclerosing panencephalitis;
• aseptic meningitis;
• thrombocytopenia;
• aplastic anemia (anemia due to the bone marrow’s inability to produce platelets, red and white blood cells);
• lymphadenitis (inflammation of the lymph nodes);
• anaphylaxis including related symptoms of peripheral, angioneurotic and facial edema;
• agitation;
• ocular palsies;
• necrotizing retinitis (inflammation of the eye);
• nerve deafness;
• optic and retrobulbar neuritis (inflammation of the optic nerve);
• Bell’s palsy (sudden but temporary weakness of one half of the face);
• cerebrovascular accident (stroke);
• acute disseminated encephalomyelitis;
• measles inclusion body encephalitis;
• transverse myelitis;
• encephalopathy;
• Guillain-Barré syndrome;
• syncope (fainting);
• tremor;
• dizziness;
• paraesthesia;
• febrile seizure;
• afebrile seizures or convulsions;
• polyneuropathy (dysfunction of numerous peripheral nerves of the body);
• Stevens-Johnson syndrome;
• Henoch-Schönlein purpura;
• acute hemorrhagic edema of infancy;
• erythema multiforme;
• panniculitis;
• arthritis;
• death.
A 2014 published study on the MMR-V vaccine in Canada determined that the risk of febrile seizures to be double in children receiving the MMR-V vaccine when compared to those receiving the MMR and varicella vaccines separately.
A 2015 meta-analysis found a two-fold increase in febrile seizures between 5 and 12 days or 7 and 10 days following MMR-V vaccination in children between the ages of 10 and 24 months.
MMR-V vaccine contains albumin, a human blood derivative, and as a result, a theoretical risk of contamination with Creutzfeldt-Jakob disease (CJD) exists.
Merck states that no cases of transmission of CJD or other viral diseases have been identified and virus pools, cells, bovine serum, and human albumin used in vaccine manufacturing are all tested to assure the final product is free of potentially harmful agents.
Serious complications reported by Merck in the MMRII product insert during vaccine post-marketing surveillance include:
• brain inflammation (encephalitis) and encephalopathy (chronic brain dysfunction);
• panniculitis (inflammation of the fat layer under the skin);
• atypical measles;
• syncope (sudden loss of consciousness, fainting);
• vasculitis (inflammation of the blood vessels);
• pancreatitis (inflammation of the pancreas);
• diabetes mellitus;
• thrombocytopenia purpura (blood disorder);
• Henoch-Schönlein purpura (inflammation and bleeding in the small blood vessels);
• acute hemorrhagic edema of infancy (rare vasculitis of the skin’s small vessels occurring in infants);
• leukocytosis (high white blood cell count);
• anaphylaxis (shock);
• bronchial spasms;
• pneumonia;
• pneumonitis (inflammation of the lung tissues);
• arthritis and arthralgia (joint pain);
• myalgia (muscle pain);
• polyneuritis (inflammation of several nerves simultaneously);
• measles inclusion body encephalitis (disease affecting the brain of immunocompromised persons);
• subacute sclerosing panencephalitis (fatal progressive brain disorder caused by exposure to the measles virus);
• Guillain-Barre Syndrome (GBS) (disease where the body’s immune system attacks the nerves);
• acute disseminated encephalomyelitis (ADEM) (brief widespread inflammation of the nerve’s protective covering);
• transverse myelitis (inflammation of the spinal cord);
• aseptic meningitis;
• erythema multiforme (skin disorder from an allergic reaction or infection);
• urticarial rash (hives, itching from an allergic reaction);
• measles-like rash;
• Stevens-Johnson syndrome (severe reaction causing the skin and mucous membranes to blister, die, and shed);
• nerve deafness (hearing loss from damage to the inner ear);
• otitis media (ear infection);
• retinitis (inflammation of the retina of the eye);
• optic neuritis (inflammation of the optic nerve);
• conjunctivitis (pink eye);
• ocular palsies (dysfunction of the ocular nerve);
• epididymitis (inflammation of the epididymis);
• paresthesia (burning or prickling of the skin);
• death.
In the comprehensive report evaluating scientific evidence, Adverse Effects of Vaccines: Evidence and Causality, published in 2012 by the Institute of Medicine (IOM), 30 reported vaccine adverse events following the Measles, Mumps, and Rubella (MMR) vaccine were evaluated by a physician committee.
These adverse events included measles inclusion body encephalitis, febrile seizures, arthritis, meningitis, Guillain Barre Syndrome, autism, diabetes mellitus, optic neuritis, transverse myelitis and more.
In 23 of the 30 measles, mumps, and rubella (MMR) vaccine-related adverse events evaluated, the IOM committee concluded that there was inadequate evidence to support or reject a causal relationship between the MMR vaccine and the reported adverse event, primarily because there was either an absence of methodologically sound published studies or too few quality studies to make a determination.
The IOM committee, however, concluded that the scientific evidence “convincingly supports” a causal relationship between febrile seizures, anaphylaxis, and measles inclusion body encephalitis in immunocompromised individuals and the MMR vaccine and favored acceptance of a causal relationship between transient arthralgia in both children and women and the MMR vaccine.
The IOM committee also concluded that it favored rejection of a causal association between both autism and the MMR vaccine and Type 1 diabetes and the MMR vaccine, however, both of these conclusions were made following the review of only five epidemiological studies.
In 2012, the Cochrane Collaborative examined 57 studies and clinical trials involving approximately 14.7 million children who had received the MMR vaccine.
While the study authors stated that they were not able to detect a “significant” association between MMR vaccine and autism, asthma, leukemia, hay fever, type I diabetes, gait disturbance, Crohn’s disease, demyelinating diseases or bacterial or viral infections, they reported that “The design and reporting of safety outcomes in MMR vaccine studies, both pre- and post-marketing, are largely inadequate.”
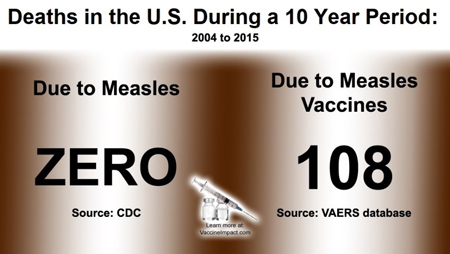
As of November 30, 2018, in the US there have been more than 93,179 reports of measles vaccine reactions, hospitalizations, injuries and deaths following measles vaccinations made to the federal Vaccine Adverse Events Reporting System (VAERS), including 459 related deaths, 6,936 hospitalizations, and 1,748 related disabilities.
Over 50% of those adverse events occurred in children three years old and under. However, the numbers of vaccine-related injuries and deaths reported to VAERS may not reflect the true number of serious health problems that occur develop after MMR vaccination.
Even though the National Childhood Vaccine Injury Act of 1986 legally required pediatricians and other vaccine providers to report serious health problems following vaccination to federal health agencies (VAERS), many doctors and other medical workers giving vaccines to children and adults fail to report vaccine-related health problem to VAERS.
There is evidence that only between one and 10 percent of serious health problems that occur after use of prescription drugs or vaccines in the U.S. are ever reported to federal health officials, who are responsible for regulating the safety of drugs and vaccines and issue national vaccine policy recommendations.
As of January, 2019, until March, 2019, there have been 1,258 claims filed in the federal Vaccine Injury Compensation Program (VICP) for 82 deaths and 1,176 injuries that occurred after measles vaccination.
Of that number, the U.S. Court of Claims administering the VICP has compensated 483 children and adults, who have filed claims for measles vaccine injury.
Read the second part of this articlehere
yogaesoteric
May 25, 2019
