Using a virus to kill what antibiotics can’t
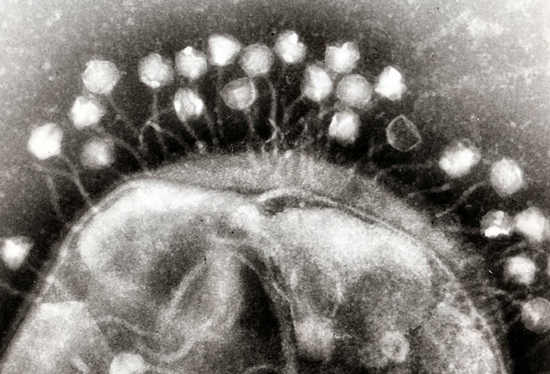
Phages on the surface of a bacterial cell
Due largely to overuse, we’re at risk of seeing many of our antibiotics lose effectiveness, leaving us without a defense against a number of potentially fatal infections. People are taking a variety of approaches to dealing with this, like looking for combinations of drugs that remain effective, developing entirely new drugs, and trying to reform how we dispense these critical drugs. (Although the latter may be an impossible dream.)
There’s another option that was under consideration even before antibiotic resistance had hit crisis levels: use something that makes killing bacteria part of its life cycle. Like other cells, bacteria often find themselves victims of viral infections, dying as new viruses burst out to infect their neighbors. If this happens out in regular ecosystems, people reasoned that maybe bacteria-killing viruses would also work in a pneumonic lung. But those “maybe-s” had always been accompanied by a long list of reasons why a virus wouldn’t work. Now, a group of researchers has tested it on mice with pneumonia, and none of those reasons seems to be an issue.
Meet the phages
Viruses that specialize in infecting bacteria are often called bacteriophages, or simply phages. We’ve known of some of them from shortly after we started studying bacteria, since their spontaneous infections would leave open holes of what would otherwise be an even lawn of bacteria. We’ve studied a number of them in detail, and some of the proteins they encode have become key tools in our genetic-engineering efforts. And they’re not simply oddities that strike when bacteria are forced to live in artificial lab conditions. Surveys of DNA obtained in environments from the deep ocean to the subways show that, wherever you find bacteria, you also find viruses that prey on them.
There are a lot of potential advantages to using phages to target bacterial infections, beyond the fact that every cell infected will produce more bacteria-killing phages. Many phages have a small range of bacterial species that they can infect, so they’re less likely to wipe out all the other bacteria that are normally part of the ecosystem that lives in or on our bodies and seem to generally contribute to human health. There are also far, far more viruses that we haven’t characterized yet, meaning that we’re less likely to suddenly run out of options if a few phages stop working. And finally, combining several phage species into a single treatment is also possible, making it much harder for bacteria to evolve resistance.
All of which sounds possible – so why aren’t we using them? “Unlikely to evolve resistance” doesn’t help much if bacteria have run into a particular phage previously and have already evolved resistance. But that’s not the end of the problems. Phages are much bigger than antibiotics and won’t simply diffuse to the site of an infection. And the immune system is primed to attack and clear out things that look like phages, so it’s likely to attack them as well.
Delivery vehicles
Despite these potential problems, a US-based team of researchers decided to tackle the delivery problem. The team chose the lung as the target tissue, since drug-resistant pneumonia represents a significant threat. This involved a bit of a balancing act. Particles had to be small and low density to ensure that they’d penetrate deep into the lung when inhaled. But they couldn’t be too small or they’d end up being swallowed by macrophages, white blood cells that help the immune system clean out infections.
To manage this balancing act, the researchers relied on a technique that creates shells of oil that surround a small bubble of water while floating in a water solution. The oil contained the building blocks of a biodegradable polymer. And the water contained a chemical that would slowly decompose into gases, disturbing the surface of the oil and making for a rough polymer shell. By carefully controlling the oil-bubble formation, the researchers could control the size of the polymer shells that were produced, making them the right size to get deep into the lung.
To get these shells to carry phage, the researchers simply soaked them in a solution of phage. They could then be dried and stored for a few weeks without losing much activity. The dry powder could then be inhaled to deliver the phage into the lungs.
From there on out, most of the work involves confirming that the phages are still capable of killing Pseudomonas aeruginosa, a cause of drug-resistant lung infections. The treatment worked in culture dishes and when the bacteria were given the chance to form a biofilm, a dense, three-dimensional mesh that can protect bacteria from drugs. (It also worked on what the researchers term “synthetic sputum”, but we chose not to look into the details there.)
Animal testing
So, the researchers went on to try their treatment in live mice they had infected with P. aeruginosa. Use of the virus-covered particles dropped infection levels by a factor of 10, which appeared to be enough to allow the immune system to get control over the infection. While only 13 percent of untreated mice survived the infection, all of the phage-treated mice survived. The treatment was also highly effective at cutting down on bacterial infections in mice lacking the gene that’s damaged in people who suffer from cystic fibrosis, which frequently results in lung infections.
To get an added degree of relevance, the researchers obtained samples from patients with pneumonia and patients with cystic fibrosis. First, they screened the samples against a library of different phages, identifying three phage species that could kill each of the infection samples. Mice were then infected and treated with the phage-covered particles. All but one of the infections were knocked down by the treatment.
Checking the mice following treatment, the researchers found that the particles themselves were gone from the lungs within 18 hours of the treatment. And there was no indication of an immune response to any of the phages. In addition, bacterial samples taken following the treatment showed no sign of containing bacteria that were resistant to phage infection. In short, none of the obvious problems that we’d expect from phage treatment seemed to be occurring.
All of which looks incredibly promising – it’s somewhat surprising that there’s no disclosure that a clinical trial is planned or underway. And the researchers indicate that things could be even better, as phages can be engineered to carry enzymes that make the bacteria even more vulnerable (by digesting biofilms, as one example). There are still some details we’ll want to understand better, like why one bacterial sample was able to do fine despite the phage, but these initial results make getting the details seem like a priority.
yogaesoteric
October 21, 2018
Also available in:
 Français
Français
